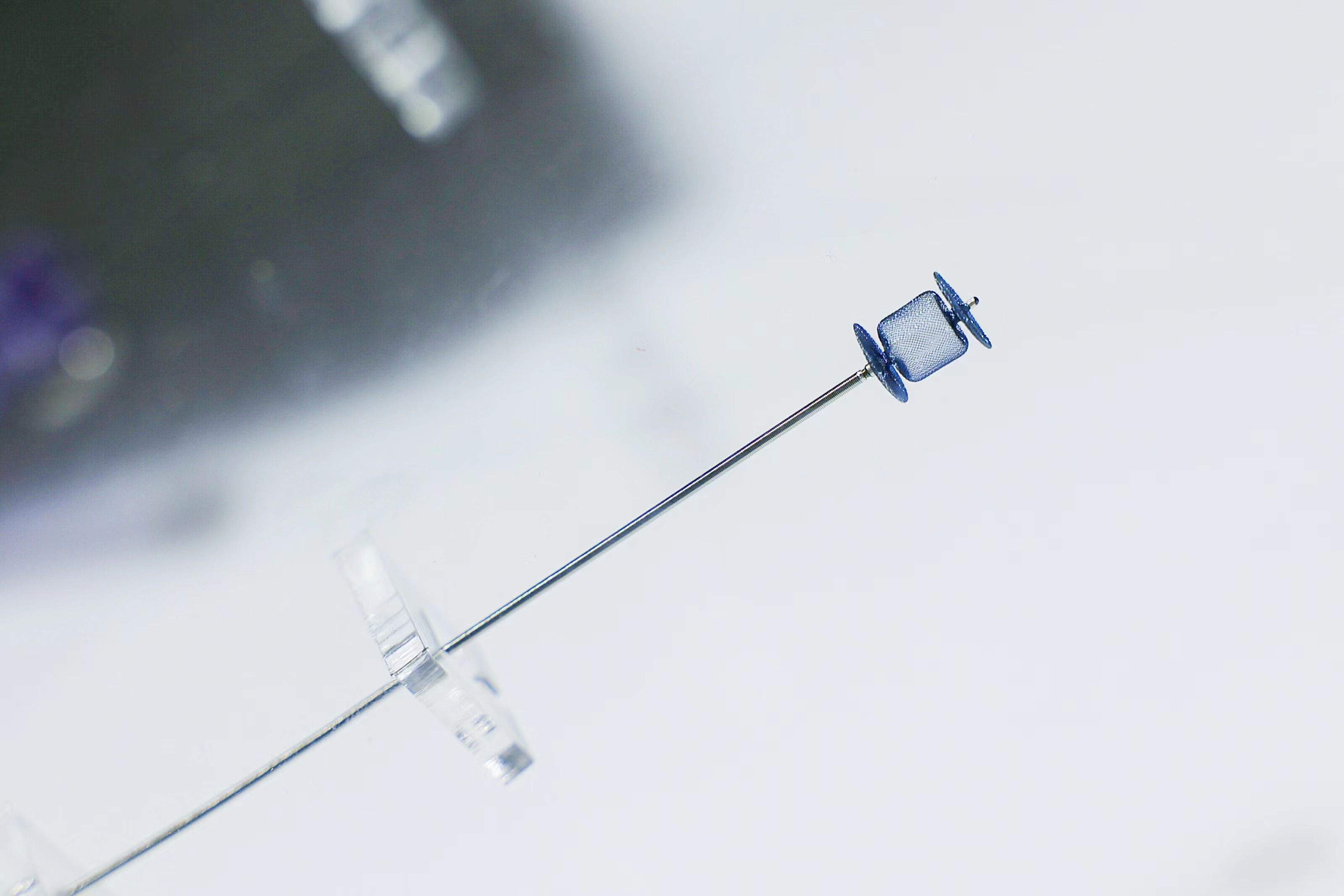
Piccolo Amplatzer . The amplatzer piccolo, a device even smaller than a small pea, now offers hope to premature infants and newborns who need. The amplatzer piccolo™ occluder, is designed to occlude small ducts in patients 700g and up, including neonates and infants. Advancing a proven platform for predictable results. This study supports the safety and effectiveness of the amplatzer piccolo occluder, particularly in patients between 700 g and 2 kg where there is. The device can be safely implanted to help seal the. The amplatzer piccolo occluder is a treatment for the congenital heart defect known as patent ductus arteriosus (pda). The amplatzer piccolo™ occluder, which delivers proven pda closure for patients 700 g and up, provides the strength to occlude small ducts,. The amplatzer piccolo™ occluder is the only minimally invasive pda closure device that is fda approved for premature infants.
from 3w.huanqiu.com
The amplatzer piccolo, a device even smaller than a small pea, now offers hope to premature infants and newborns who need. This study supports the safety and effectiveness of the amplatzer piccolo occluder, particularly in patients between 700 g and 2 kg where there is. The amplatzer piccolo™ occluder, which delivers proven pda closure for patients 700 g and up, provides the strength to occlude small ducts,. The amplatzer piccolo occluder is a treatment for the congenital heart defect known as patent ductus arteriosus (pda). The device can be safely implanted to help seal the. The amplatzer piccolo™ occluder, is designed to occlude small ducts in patients 700g and up, including neonates and infants. Advancing a proven platform for predictable results. The amplatzer piccolo™ occluder is the only minimally invasive pda closure device that is fda approved for premature infants.
雅培携多款创新解决方案亮相第五届进博会 “首秀”硬核产品重磅登场
Piccolo Amplatzer The amplatzer piccolo™ occluder, is designed to occlude small ducts in patients 700g and up, including neonates and infants. Advancing a proven platform for predictable results. The amplatzer piccolo™ occluder is the only minimally invasive pda closure device that is fda approved for premature infants. This study supports the safety and effectiveness of the amplatzer piccolo occluder, particularly in patients between 700 g and 2 kg where there is. The device can be safely implanted to help seal the. The amplatzer piccolo™ occluder, is designed to occlude small ducts in patients 700g and up, including neonates and infants. The amplatzer piccolo™ occluder, which delivers proven pda closure for patients 700 g and up, provides the strength to occlude small ducts,. The amplatzer piccolo, a device even smaller than a small pea, now offers hope to premature infants and newborns who need. The amplatzer piccolo occluder is a treatment for the congenital heart defect known as patent ductus arteriosus (pda).
From health.economictimes.indiatimes.com
Abbott launches Amplatzer Piccolo in India for treatment of heart Piccolo Amplatzer The amplatzer piccolo, a device even smaller than a small pea, now offers hope to premature infants and newborns who need. Advancing a proven platform for predictable results. The device can be safely implanted to help seal the. The amplatzer piccolo™ occluder is the only minimally invasive pda closure device that is fda approved for premature infants. This study supports. Piccolo Amplatzer.
From onlinelibrary.wiley.com
Amplatzer Piccolo Occluder clinical trial for percutaneous closure of Piccolo Amplatzer The amplatzer piccolo occluder is a treatment for the congenital heart defect known as patent ductus arteriosus (pda). The amplatzer piccolo™ occluder is the only minimally invasive pda closure device that is fda approved for premature infants. Advancing a proven platform for predictable results. The device can be safely implanted to help seal the. The amplatzer piccolo™ occluder, which delivers. Piccolo Amplatzer.
From www.structuralheart.abbott
PDA Closure in infants Piccolo Amplatzer The amplatzer piccolo™ occluder is the only minimally invasive pda closure device that is fda approved for premature infants. The amplatzer piccolo, a device even smaller than a small pea, now offers hope to premature infants and newborns who need. The amplatzer piccolo™ occluder, is designed to occlude small ducts in patients 700g and up, including neonates and infants. The. Piccolo Amplatzer.
From www.cureus.com
Cureus Transcatheter Closure of a Patent Ductus Arteriosus Using a Piccolo Amplatzer Advancing a proven platform for predictable results. The amplatzer piccolo occluder is a treatment for the congenital heart defect known as patent ductus arteriosus (pda). This study supports the safety and effectiveness of the amplatzer piccolo occluder, particularly in patients between 700 g and 2 kg where there is. The amplatzer piccolo™ occluder is the only minimally invasive pda closure. Piccolo Amplatzer.
From www.bibamedtech.com
CE marks for Masters HP 15mm and Amplatzer Piccolo Occluder BIBA Piccolo Amplatzer This study supports the safety and effectiveness of the amplatzer piccolo occluder, particularly in patients between 700 g and 2 kg where there is. The device can be safely implanted to help seal the. The amplatzer piccolo occluder is a treatment for the congenital heart defect known as patent ductus arteriosus (pda). The amplatzer piccolo™ occluder, is designed to occlude. Piccolo Amplatzer.
From structuralheart.insidepractice.com.au
Patent Ductus with the Amplatzer Piccolo™ Occluder Inside Practice Piccolo Amplatzer Advancing a proven platform for predictable results. The device can be safely implanted to help seal the. This study supports the safety and effectiveness of the amplatzer piccolo occluder, particularly in patients between 700 g and 2 kg where there is. The amplatzer piccolo, a device even smaller than a small pea, now offers hope to premature infants and newborns. Piccolo Amplatzer.
From expomedical.ru
Amplatzer Piccolo Окклюдер кардиологический Abbott Medical Piccolo Amplatzer The amplatzer piccolo™ occluder, which delivers proven pda closure for patients 700 g and up, provides the strength to occlude small ducts,. The device can be safely implanted to help seal the. The amplatzer piccolo occluder is a treatment for the congenital heart defect known as patent ductus arteriosus (pda). This study supports the safety and effectiveness of the amplatzer. Piccolo Amplatzer.
From expomedical.ru
Amplatzer Piccolo Abbott Medical (США) Piccolo Amplatzer The amplatzer piccolo™ occluder is the only minimally invasive pda closure device that is fda approved for premature infants. Advancing a proven platform for predictable results. This study supports the safety and effectiveness of the amplatzer piccolo occluder, particularly in patients between 700 g and 2 kg where there is. The amplatzer piccolo, a device even smaller than a small. Piccolo Amplatzer.
From www.medicaldevice-network.com
Amplatzer Piccolo Occluder Device for Patent Ductus Arteriosus, USA Piccolo Amplatzer The device can be safely implanted to help seal the. The amplatzer piccolo™ occluder, which delivers proven pda closure for patients 700 g and up, provides the strength to occlude small ducts,. This study supports the safety and effectiveness of the amplatzer piccolo occluder, particularly in patients between 700 g and 2 kg where there is. The amplatzer piccolo occluder. Piccolo Amplatzer.
From www.medicaldevice-network.com
Abbott's Amplatzer Piccolo Occluder for babies gets FDA approval Piccolo Amplatzer The device can be safely implanted to help seal the. The amplatzer piccolo™ occluder, is designed to occlude small ducts in patients 700g and up, including neonates and infants. This study supports the safety and effectiveness of the amplatzer piccolo occluder, particularly in patients between 700 g and 2 kg where there is. The amplatzer piccolo™ occluder, which delivers proven. Piccolo Amplatzer.
From www.researchgate.net
(PDF) Percutaneous patent ductus arteriosus closure in extremely low Piccolo Amplatzer The device can be safely implanted to help seal the. The amplatzer piccolo, a device even smaller than a small pea, now offers hope to premature infants and newborns who need. The amplatzer piccolo™ occluder, is designed to occlude small ducts in patients 700g and up, including neonates and infants. The amplatzer piccolo™ occluder is the only minimally invasive pda. Piccolo Amplatzer.
From sufomeca.com
Dispositivo Oclusor Ductus PDA Amplatzer Solución Avanzada Sufomeca Piccolo Amplatzer The amplatzer piccolo™ occluder, is designed to occlude small ducts in patients 700g and up, including neonates and infants. The amplatzer piccolo, a device even smaller than a small pea, now offers hope to premature infants and newborns who need. Advancing a proven platform for predictable results. The amplatzer piccolo occluder is a treatment for the congenital heart defect known. Piccolo Amplatzer.
From 3w.huanqiu.com
雅培携多款创新解决方案亮相第五届进博会 “首秀”硬核产品重磅登场 Piccolo Amplatzer The amplatzer piccolo, a device even smaller than a small pea, now offers hope to premature infants and newborns who need. The amplatzer piccolo occluder is a treatment for the congenital heart defect known as patent ductus arteriosus (pda). The amplatzer piccolo™ occluder is the only minimally invasive pda closure device that is fda approved for premature infants. The device. Piccolo Amplatzer.
From medicaldialogues.in
Abbott launches Amplatzer Piccolo Occluder in India to treat congenital Piccolo Amplatzer Advancing a proven platform for predictable results. The amplatzer piccolo™ occluder is the only minimally invasive pda closure device that is fda approved for premature infants. The device can be safely implanted to help seal the. The amplatzer piccolo™ occluder, is designed to occlude small ducts in patients 700g and up, including neonates and infants. The amplatzer piccolo, a device. Piccolo Amplatzer.
From www.linkedin.com
Abbott's Amplatzer Piccolo Occluder a heart device for newborns shows Piccolo Amplatzer The amplatzer piccolo, a device even smaller than a small pea, now offers hope to premature infants and newborns who need. The device can be safely implanted to help seal the. The amplatzer piccolo™ occluder is the only minimally invasive pda closure device that is fda approved for premature infants. The amplatzer piccolo™ occluder, which delivers proven pda closure for. Piccolo Amplatzer.
From www.structuralheart.abbott
Amplatzer Piccolo Occluder Piccolo Amplatzer The amplatzer piccolo, a device even smaller than a small pea, now offers hope to premature infants and newborns who need. The amplatzer piccolo™ occluder is the only minimally invasive pda closure device that is fda approved for premature infants. The device can be safely implanted to help seal the. The amplatzer piccolo occluder is a treatment for the congenital. Piccolo Amplatzer.
From www.rdworldonline.com
Amplatzer Piccolo Occluder Research & Development World Piccolo Amplatzer The amplatzer piccolo™ occluder, is designed to occlude small ducts in patients 700g and up, including neonates and infants. The amplatzer piccolo™ occluder is the only minimally invasive pda closure device that is fda approved for premature infants. This study supports the safety and effectiveness of the amplatzer piccolo occluder, particularly in patients between 700 g and 2 kg where. Piccolo Amplatzer.
From corpmedical.cl
Abbott AMPLATZER™ PDA Corpmedical Piccolo Amplatzer Advancing a proven platform for predictable results. This study supports the safety and effectiveness of the amplatzer piccolo occluder, particularly in patients between 700 g and 2 kg where there is. The device can be safely implanted to help seal the. The amplatzer piccolo™ occluder, is designed to occlude small ducts in patients 700g and up, including neonates and infants.. Piccolo Amplatzer.